Corticosteroid vs Autologous
Protein Solution
About
Shoulder pain accounts for 1–2% of all adult consultations with a GP in the UK. Despite being a common injury, shoulder pain does not always have a favourable outcome with current treatments.
Currently the most common treatments are steroid injections combined with physiotherapy. Recently, there have been questions about whether using steroid injections is actually effective in the long term, or if there are any negative effects on the nearby tendons.
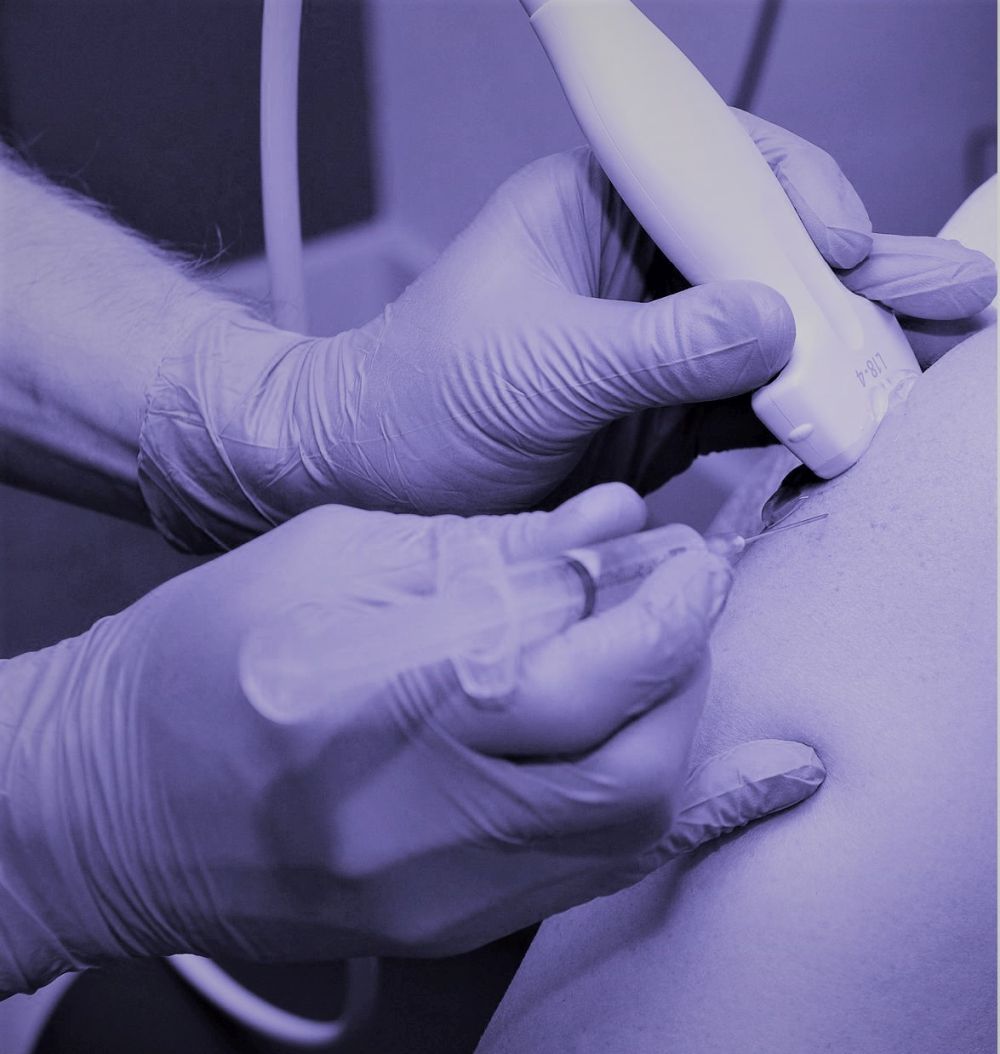
This has led to the development of new injectable treatments aimed to help tendons repair themselves. These are termed 'biologic' injections, and are produced from the patients own blood.
The aim of our study is to see if it is feasible to compare one of these biologic-injection treatments against steroid injections.
Who can participate?
All adults, 18 years and older who have symptoms suggestive of subacromial pain syndrome and would normally be offered CorticoSteroid injections treatment for their injury.
What does the study involve?
50 participants will be recruited to the trial. Half will receive CorticoSteroid injections treatment and half will receive a 'biologic' injection. All participants will be asked for a sample of blood. This blood will be spun to create the Autologous Protein Solution for the ‘biologic’ injection treatment. Those participants receiving CorticoSteroid injection will have their blood sample discarded, this is to ensure that participants will not know which injection they have received.
What are the possible benefits and risks of participating?
Both treatments are used across the NHS so there is no specific advantage to taking part in the study, however, participation will help us improve treatment for future patients with similar pain. After receiving either injection, some participants may experience mild to moderate pain local to the injection site. The rare risk of infection following an injection for both treatments is the same.
Where is the study run from?
The University of Oxford is the lead centre for the study, and the day to day running of the study is being completed by Oxford Trauma and Emergency Care team within the Oxford Clinical Trials Research Unit.
When is the study starting and how long is it expected to run for?
Recruitment of participants is expected to start in March 2022. This recruitment period will end 6 months later in September 2022. The results of the study will then be analysed and published, and the study will be completed by January 2023.
Who is funding the study?
The study is funded by the National Institute for Health Research (NIHR) – Research for Patient Benefit (RfPB).
Your Data
Your personal details will be held by the research team at University of Oxford. We will remove any details that would identify you personally (such as your name, date of birth, etc.) from your answers to our questions and no individual results will be published.
Any data from which you can be identified, such as your name, gender, date of birth, or data concerning your health, is known as personal data. The University of Oxford, as sponsor, is the data controller. This means that the University of Oxford is responsible for looking after your information and using it properly. We will use the minimum personally-identifiable information possible.
We will keep any research documents with identifiable information about you for 12 months after the study has finished. Your contact details will be kept until the follow-up is completed. We will store the anonymised research data securely at the University of Oxford for a minimum of three years after publication of the results of the study. You have the right to access, change or remove your contact details. Your rights to access, change or remove your NHS number (or equivalent), gender, date of birth and postcode, and data concerning your health, are limited, as we need to manage your information in specific ways in order for the research to be reliable and accurate, and restricting its use would seriously impair its use for research purposes.
Your personal data will only be used as we have explained on this page, and research is a task that we perform in the public interest. If you are concerned about how your personal data is being used, please contact the study team using the contact details on the Contact page.
Data protection regulation provides you with control over your personal data and how it is used. When you agree to your information being used in research, however, some of those rights may be limited in order for the research to be reliable and accurate. Further information about your rights with respect to your personal data is available at https://compliance.admin.ox.ac.uk/research-data .
The lawful basis for the processing of your personal data is governed by the General Data Protection Regulation (GDPR) Articles 6 & 9. The University of Oxford will not transfer your personal data to any third countries or international organisations.
© This template is made with by ThemeWagon

